Cranial Snowflake Interlink Plate Ⅱ
Cranial Snowflake Interlink Plate II
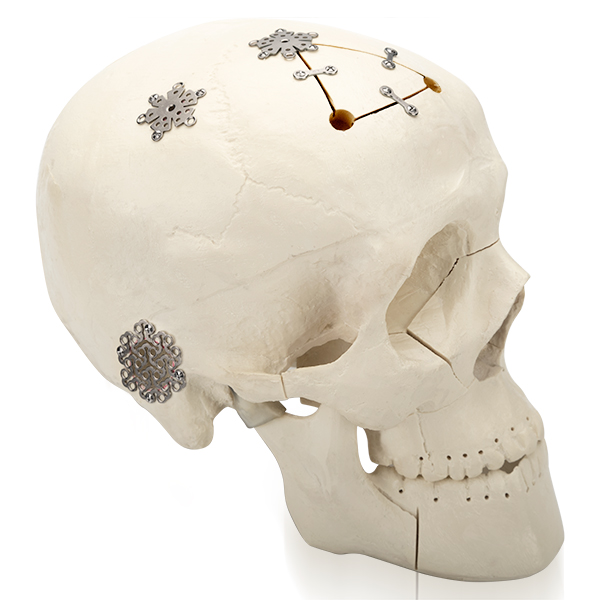
Advanced Titanium Mesh for Cranial Reconstruction
The Cranial Snowflake Interlink Plate II is specifically designed for use in neurosurgical reconstruction procedures, addressing skull defects, trauma, or congenital anomalies. This durable, lightweight mesh provides secure connection and fixation of cranial gaps, supporting both structural integrity and natural healing.
Key Applications
-
Neurosurgical skull restoration
-
Repair of cranial abnormalities
-
Fixation and reinforcement of bone gaps
Material & Design
-
Material: Medical-grade titanium
-
Thickness: 0.6 mm
-
Surface Options:
-
Non-anodized (Ref: 12.30.4010.181806)
-
Anodized (Ref: 12.30.4110.181806)
-
Features & Benefits
-
Biocompatible & Corrosion-Resistant – Suitable for long-term implantation
-
High Strength, Low Weight – Ensures stability with minimal bulk
-
Imaging Safe – No interference with CT, X-ray, or MRI
-
Supports Biological Integration – Mesh design promotes fibroblast growth and tissue bonding
-
Reliable Protection – Long-lasting performance for consistent cranial support
Compatible Screws
-
Φ1.5 mm self-drilling screw
-
Φ2.0 mm self-drilling screw